Soap Scum Chemical Reaction . If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and. A second problem is caused by the presence of calcium and. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. When these fatty acids interact with magnesium and calcium ions present in tap water. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the.
from www.numerade.com
A second problem is caused by the presence of calcium and. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Soap is primarily made of fatty acids. A second problem is caused by the. A second problem is caused by the presence of calcium and. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When these fatty acids interact with magnesium and calcium ions present in tap water. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum.
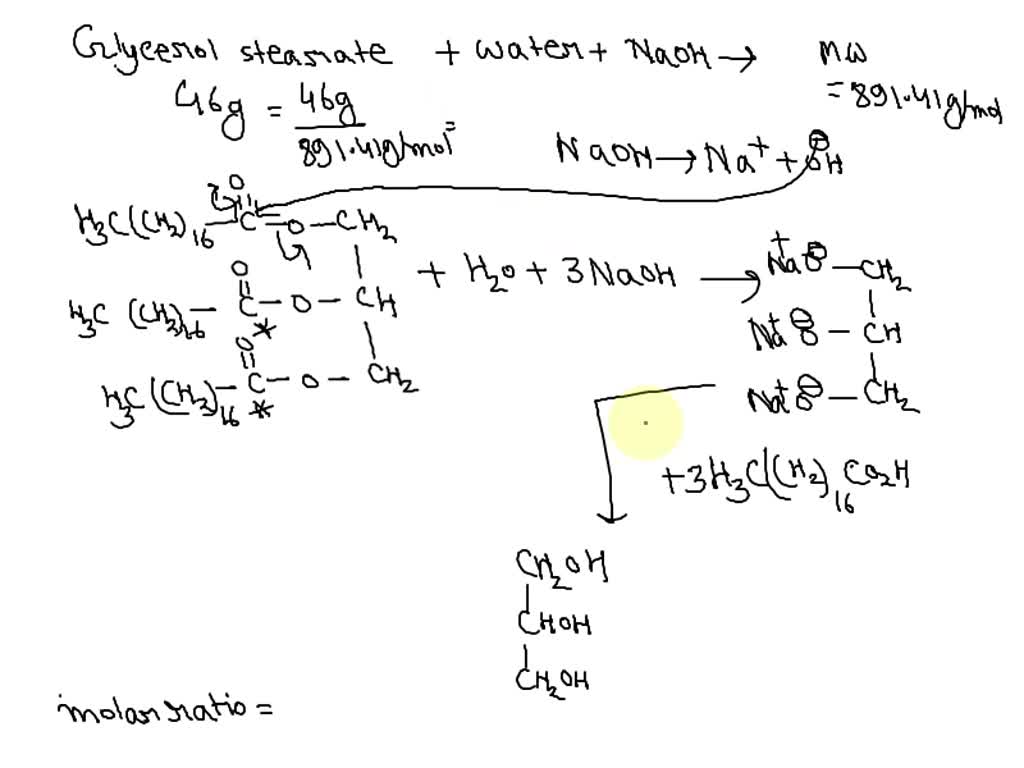
SOLVED Why was ethanol added to the reaction mixture of lard and
Soap Scum Chemical Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. A second problem is caused by the presence of calcium and. A second problem is caused by the. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and. When these fatty acids interact with magnesium and calcium ions present in tap water. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum.
From aliceinchemiland.blogspot.my
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Soap Scum Chemical Reaction When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When these fatty acids interact with magnesium and calcium ions present in tap water. Soap is primarily. Soap Scum Chemical Reaction.
From www.sciencephoto.com
Soap and hard water Stock Image C018/0069 Science Photo Library Soap Scum Chemical Reaction If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When these fatty acids interact with magnesium and calcium ions present in tap water. A second problem is caused by the presence of calcium and. A second problem is caused by the presence of calcium and. When soap dissolves in. Soap Scum Chemical Reaction.
From www.masterwater.com
The and Aesthetics of Soft Water Soap Scum Chemical Reaction A second problem is caused by the presence of calcium and. A second problem is caused by the presence of calcium and. When these fatty acids interact with magnesium and calcium ions present in tap water. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When soap dissolves in. Soap Scum Chemical Reaction.
From www.numerade.com
SOLVED Why was ethanol added to the reaction mixture of lard and Soap Scum Chemical Reaction A second problem is caused by the. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and. When these fatty acids interact with magnesium and calcium ions present in tap water. A second problem is caused by the presence. Soap Scum Chemical Reaction.
From scienceexperimentsanimated.blogspot.com
Science fair Experiments Soaps and detergents for std 8 to 10 Science Soap Scum Chemical Reaction If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. A second problem is caused by. Soap Scum Chemical Reaction.
From www.organicchem.org
soapbg Soap Scum Chemical Reaction If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and. A second problem is caused by the. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. If the ph of. Soap Scum Chemical Reaction.
From www.pinterest.com
Soft water, Hard water, Water me Soap Scum Chemical Reaction When these fatty acids interact with magnesium and calcium ions present in tap water. A second problem is caused by the. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. If. Soap Scum Chemical Reaction.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Soap Scum Chemical Reaction If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. In this collection of activities, students develop their understanding of key chemical ideas. Soap Scum Chemical Reaction.
From www.youtube.com
Cleansing action of soap Chemical reactions Chemistry YouTube Soap Scum Chemical Reaction A second problem is caused by the. When these fatty acids interact with magnesium and calcium ions present in tap water. A second problem is caused by the presence of calcium and. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. If the ph of a soap solution is. Soap Scum Chemical Reaction.
From candjwater.com
Why is there Soap Scum in my Bathtub? c and j water Soap Scum Chemical Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. When these fatty acids interact with magnesium and calcium ions present in tap water. A second problem is caused. Soap Scum Chemical Reaction.
From www.teachoo.com
Explain the formation of scum when hard water is treated with soap. Soap Scum Chemical Reaction A second problem is caused by the presence of calcium and. Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap. Soap Scum Chemical Reaction.
From www.thespruce.com
How to Clean Soap Scum Off of Any Bathroom Surface Soap Scum Chemical Reaction When these fatty acids interact with magnesium and calcium ions present in tap water. A second problem is caused by the presence of calcium and. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. In this collection of activities, students develop their understanding of key chemical ideas relating to. Soap Scum Chemical Reaction.
From www.slideserve.com
PPT CHAPTER 7 Chemical Reactions An Introduction PowerPoint Soap Scum Chemical Reaction Soap is primarily made of fatty acids. A second problem is caused by the presence of calcium and. A second problem is caused by the presence of calcium and. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. If the ph of a soap solution is lowered by acidic. Soap Scum Chemical Reaction.
From brainly.in
what is scum ? diff b\w soaps and detergents Brainly.in Soap Scum Chemical Reaction Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. When these fatty acids interact with magnesium and calcium ions present in tap water. A. Soap Scum Chemical Reaction.
From www.youtube.com
SOAPS AND DETERGENTS। FORMATION OF MICELLES। FORMATION OF SCUM Soap Scum Chemical Reaction Soap is primarily made of fatty acids. A second problem is caused by the. In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. When these fatty acids interact with magnesium and calcium ions present in tap water. When soap dissolves in water, a positive sodium ion is released into the water. Soap Scum Chemical Reaction.
From www.youtube.com
Explain the formation of scum when hard water is treated with soap Soap Scum Chemical Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and. A second problem is. Soap Scum Chemical Reaction.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2956417 Soap Scum Chemical Reaction A second problem is caused by the presence of calcium and. Soap is primarily made of fatty acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. When these fatty acids interact with magnesium and calcium ions present in tap water. When soap dissolves in water, a positive sodium. Soap Scum Chemical Reaction.
From guidetech.pages.dev
Ph Of Detergent Acidic Or Basic guidetech Soap Scum Chemical Reaction In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. When soap dissolves in water, a positive sodium ion is released into the water leaving a negatively charged end of the soap molecule. A second problem is caused by the presence of calcium and. A second problem is caused by the presence. Soap Scum Chemical Reaction.